EVO ICL Linser
STAAR Surgicals phakic IOL för närsynthet med och utan astigmatism

- begränsar behovet av preoperativa perifera iridotomier (1)
- Minskar risken för pupillblockad och grå starr (1)
- Minskar antalet återbesök för patienten
These materials are intended for medical education. By proceeding, you certify that you are a licensed health care professional.
Ett spännande nytt kapitel inom refraktiv kirurgi
The EVO ICL is a phakic intraocular and reversible implant. It is a clinically proven implantable lens that corrects common vision problems such as nearsightedness with or without astigmatism.
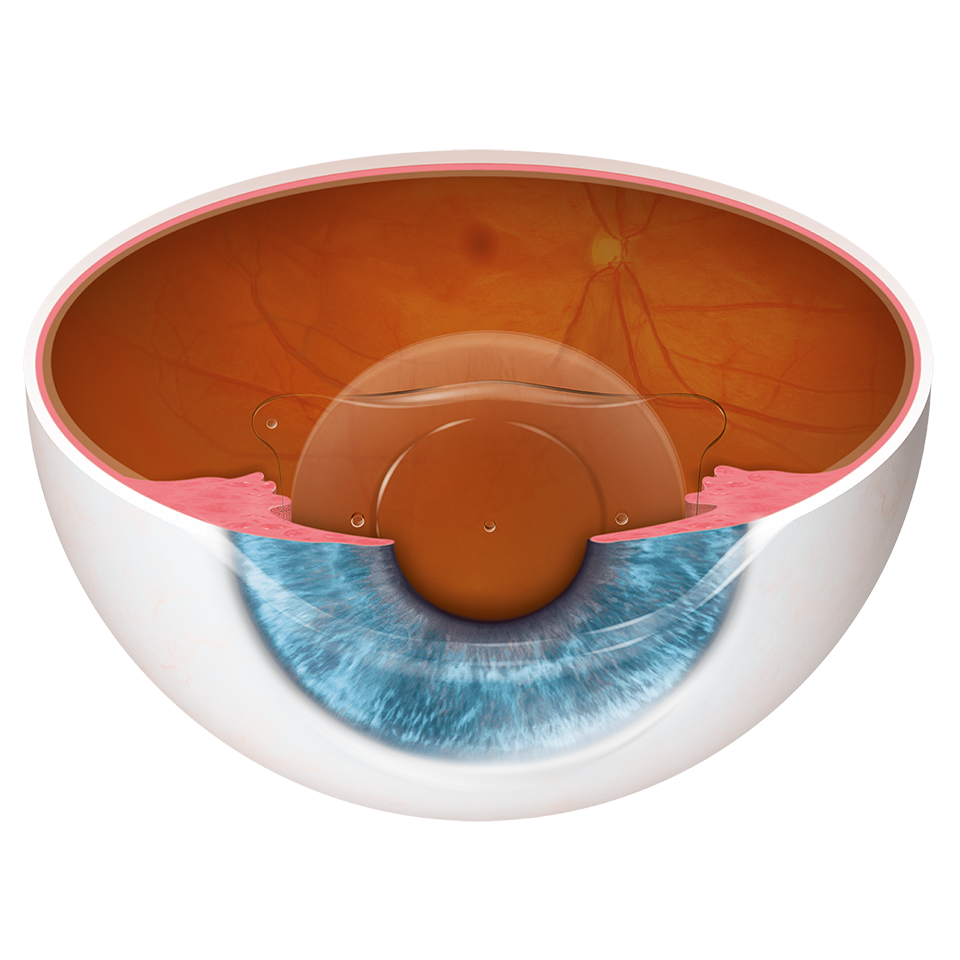
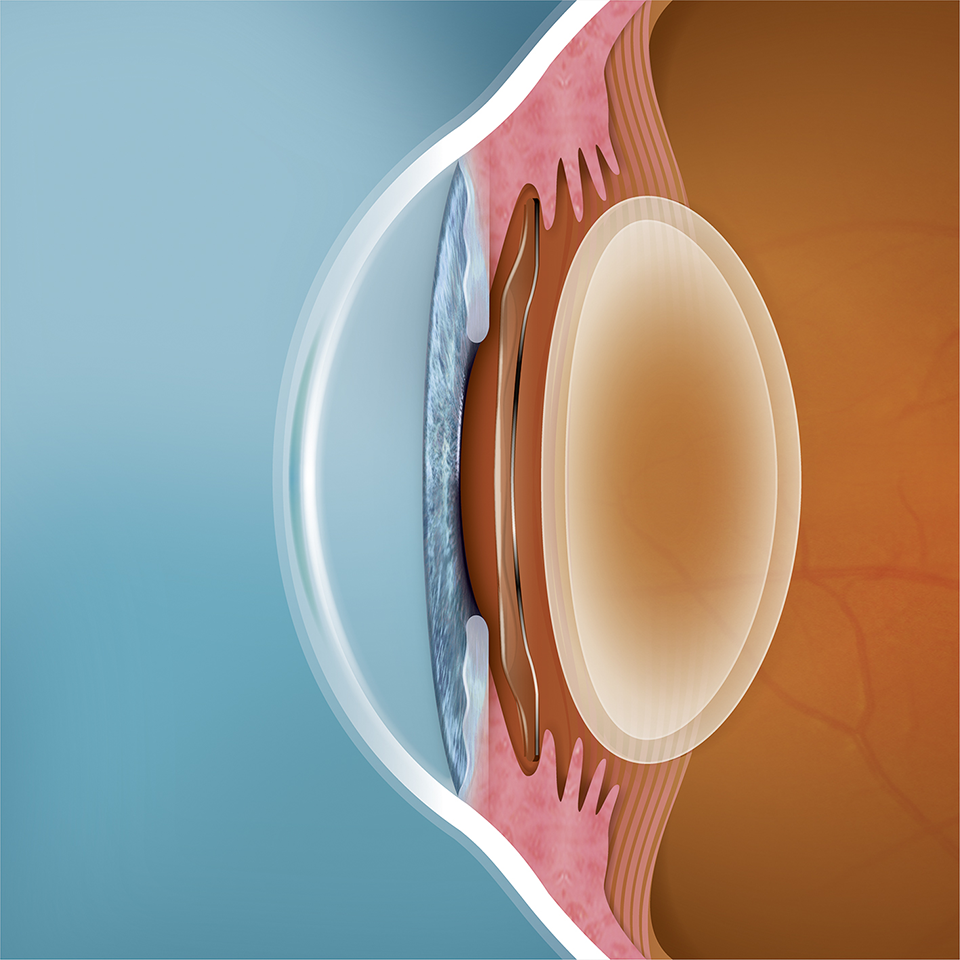
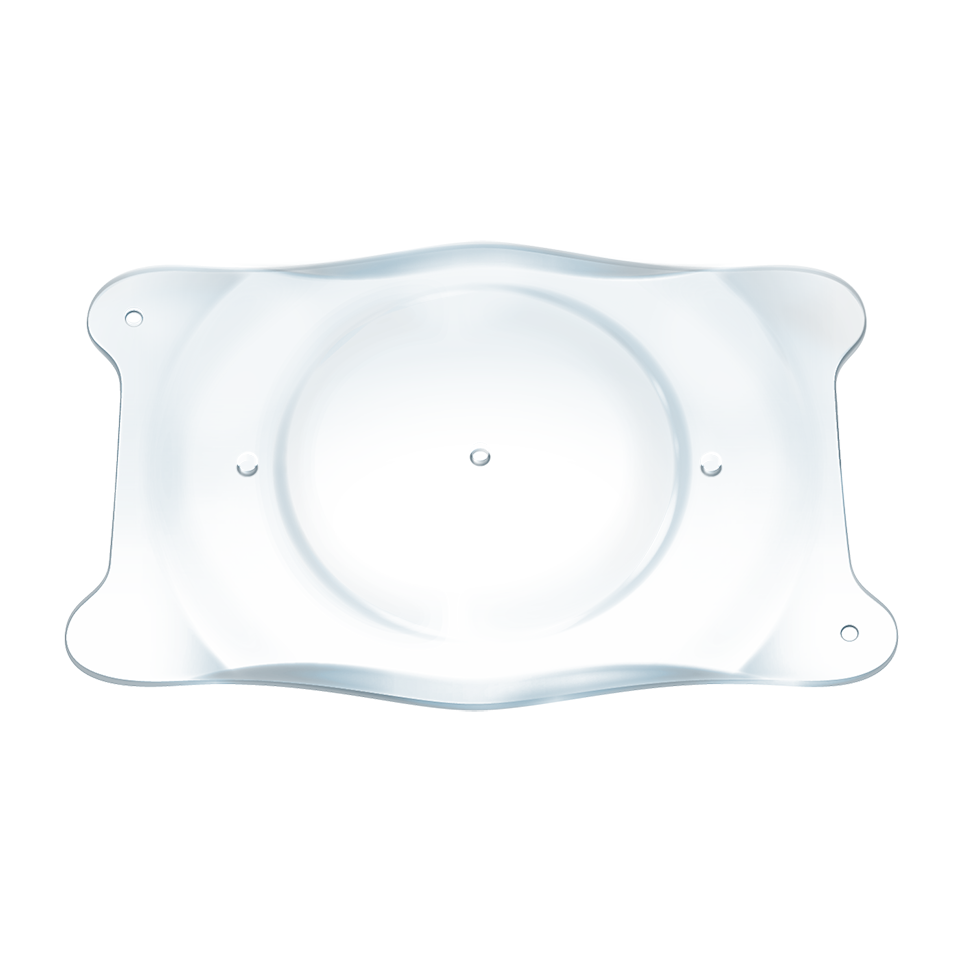
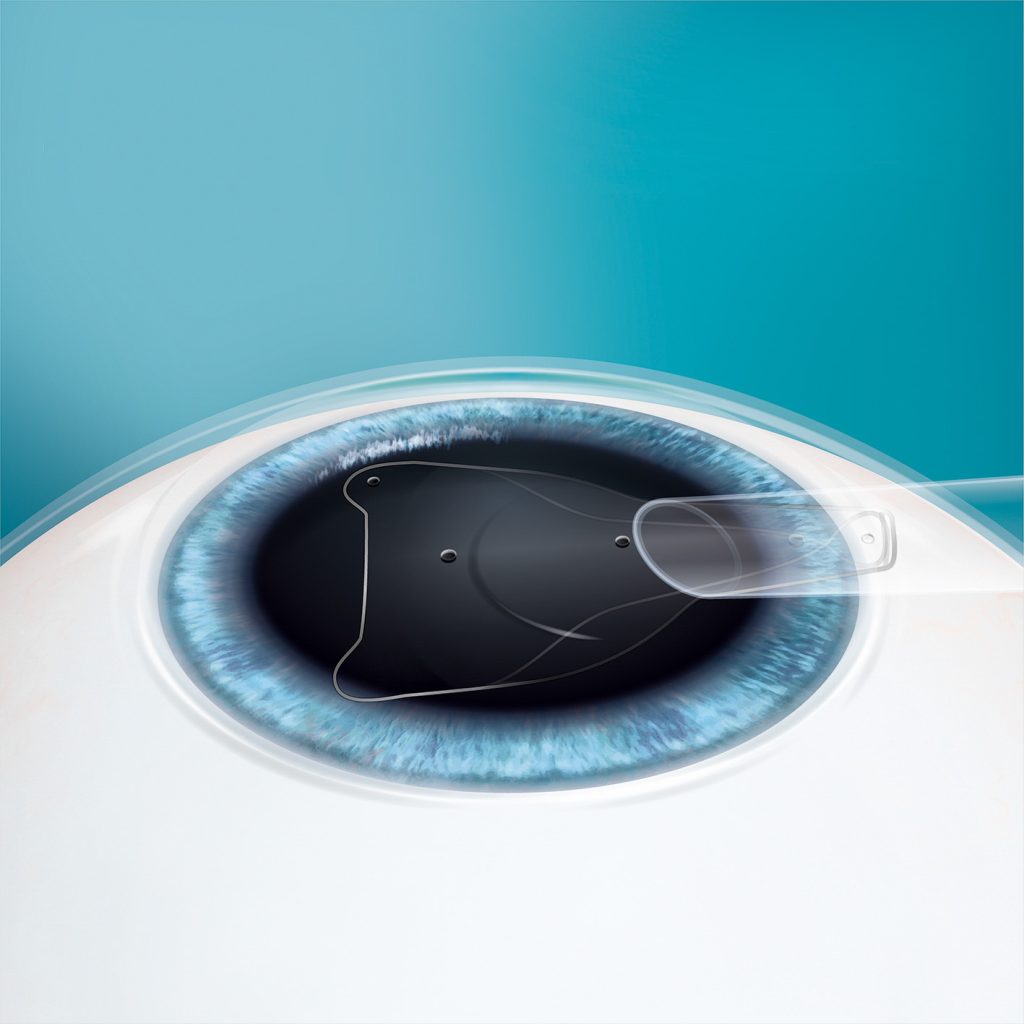
The EVO ICL is placed directly behind the iris and in front of the natural crystalline lens. The addition of the central port to EVO facilitates the flow of aqueous humor through the lens.
The EVO ICL procedure does not require the removal of any corneal tissue. Its material Collamer® is poly-HEMA-based copolymer with a UV absorbing chromophore that provides UV Protection.


- 3 000 000+ LENSES SOLD GLOBALLY
- 30 YEARS OF EXPERIENCE
- +500 SCIENTIFIC PUBLICATIONS
- +1 000 PEER-REVIEWED PUBLICATIONS
Patient selection
The EVO ICL family of lenses (EVO) is indicated for use in phakic eye treatment in patients 21 – 60 years of age who meet the criteria listed below:
- The correction/reduction of myopia with or without astigmatism
- Requiring a lens with sphere power of -0.5 D to -18.0 D and cylinder power up to +6.0 D
- With an anterior chamber depth (ACD) equal to or greater than 2.8 mm, as measured from the corneal endothelium to the anterior lens capsule
EVO uses an Exclusive, Premium Collamer® material
CollamerTM™ is the one-of-a-kind material that defines EVO ICL™ worldwide. It is a proprietary co-polymer behind the EVO ICL lens. It is a biocompatible material with properties that minimize inflamation, flare and cellular reaction.

Collamer® is a unique copolymer biocompatible material with properties that minimize inflammation, flare and cellular reaction. (8,9)

The Collamer® material is bonded with UV-absorbing chromophore into a poly-HEMA-based copolymer that offers UV protection.

Collamer® is exclusive to STAAR® Surgical. It has a proven history of over 30 years with more than 3 million ICLs distributed worldwide.
Kontakta oss rörande EVO ICL Linser
References
1. Packer M. The Implantable Collamer Lens with a central port: review of the literature. Clinical ophthalmology (Auckland, NZ). 2018;12:2427-38.
2. Kamiya K, Shimizu K, Igarashi A, Kitazawa Y, Kojima T, Nakamura T, et al. Posterior chamber phakic intraocular lens implantation: comparative, multicentre study in 351 eyes with low-to-moderate or high myopia. Br J Ophtalmol. 2018;102(2):177-81.
3. Kohnen T. Phakic intraocular lenses: Where are we now? J Cataract Refract Surg. 2018;44(2):121-3.
4. Ganesh S, Brar S, Pawar A. Matched population comparison of visual outcomes and patient satisfaction between 3 modalities for the correction of low to moderate myopic astigmatism. Clin Ophthalmol. (Auckland, NZ). 2017;11:1253-63.
5. Martínez-Plaza E, López-Miguel A, López-de la Rosa A, et al. Effect of the evo+ visian phakic implantable collamer lens on visual performance and quality of vision and life. Am J Ophthalmol. 2021;226:117-25.
6. Wei R, Li M, Zhang H, Aruma A, Miao H, Wang X, et al. Comparison of objective and subjective visual quality early after implantable collamer lens V4c (ICL V4c) and small incision lenticule extraction (SMILE) for high myopia correction. Acta Ophthalmol. 2020;98(8):e943-e50.
7. Packer M. Evaluation of the EVO/EVO+ Sphere and Toric Visian ICL: Six month results from the United States Food and Drug Administration clinical trial. Clinical Ophthalmology. 2022;16:1541-53.
8. Schild G, Amon M, Abela-Formanek C, Schauersberger J, Bartl G, Kruger A. Uveal and capsular biocompatibility of a single-piece, sharp-edged hydrophilic acrylic intraocular lens with collagen (Collamer®): 1-year results. J Cataract Refract Surg 2004;30(6):1254-8.
9. Brown DC, Ziemba SL. Collamer® intraocular lens: clinical results from the US FDA core study. J Cataract Refract Surg. 2001;27(6):833-40. 10. Igarashi A. Posterior chamber phakic iols vs. Lasik: Benefits and complications. Expert Review of Ophthalmology. 2019;14(1):43-52.
Important Safety Information for the EVO/EVO+ ICL
All physicians must complete the STAAR Surgical Visian ICL Physician Training Certification Program prior to using the EVO/EVO+ ICL in a clinical setting. Please review the EVO/EVO+ ICL Directions For Use (DFU) completely before performing a clinical procedure. INDICATIONS: The EVO/EVO+ ICL is indicated for phakic patients 21-60 years of with an anterior chamber depth (ACD) 2.8 mm or greater to correct/reduce myopia ranging from -0.5 diopters to -20.0 diopters with up to 6.0 D of astigmatism. The EVO/EVO+ ICL is intended for placement in the posterior chamber of the eye. WARNING/PRECAUTION: Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/ benefit ratio before implanting a lens in a patient with any of the conditions described in the DFU. Prior to surgery, physicians should inform prospective patients of possible risks and benefits associated with the EVO/EVO+ ICL. ATTENTION: Reference the EVO/EVO+ ICL DFU available at edfu.staar.com for a complete listing of indications, contraindications, warnings and precautions.